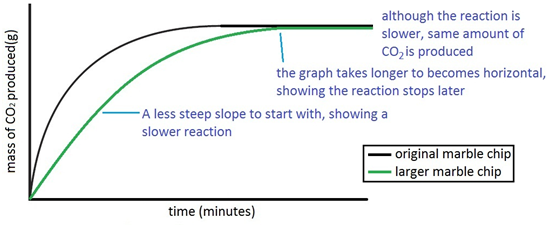
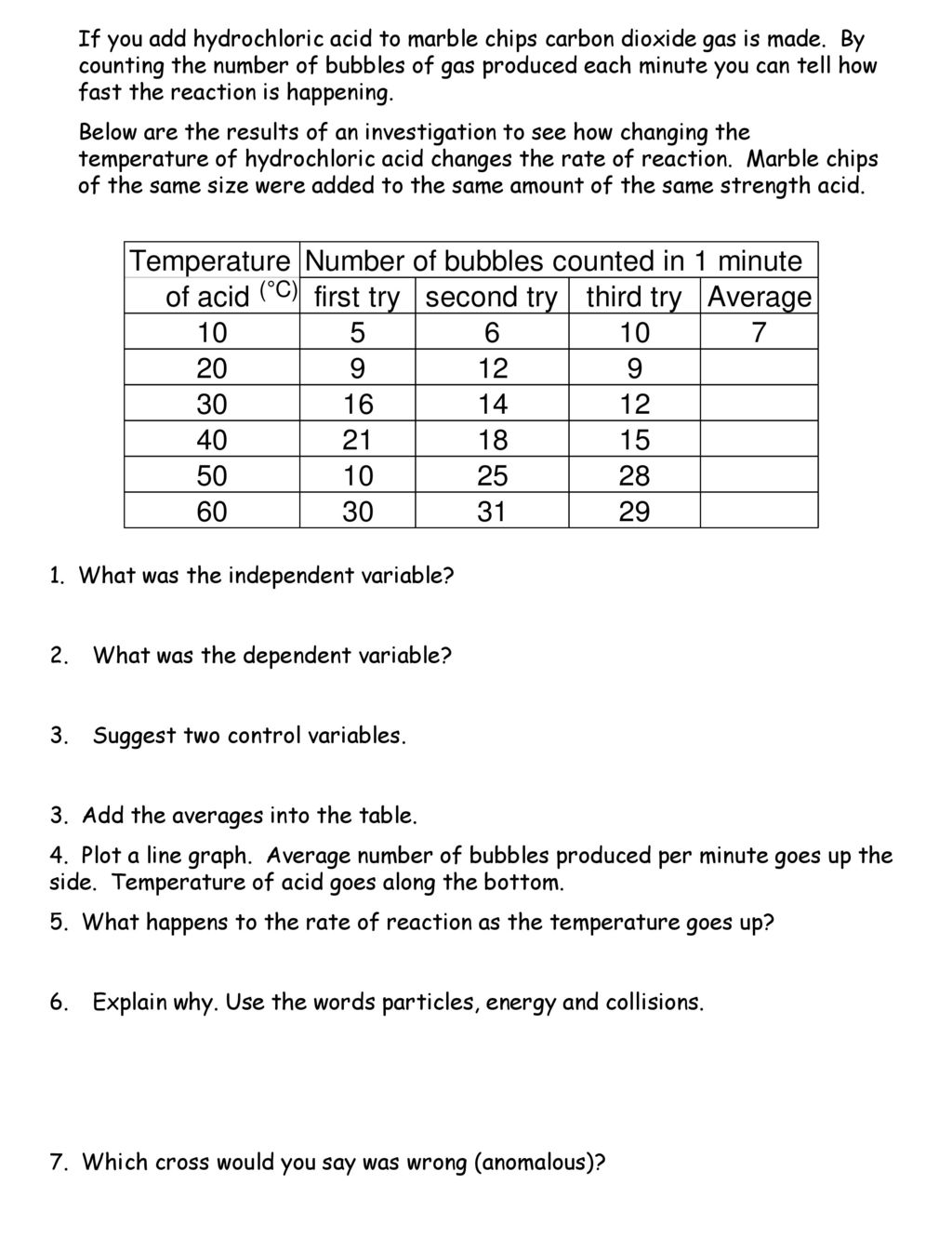
In the investigation i am going to find out how the surface area affects the rate of reaction by measuring the amount of gas produced and weight loss in a reaction between small large pieces of marble chips calcium carbonate and hydrochloric acid per minute.
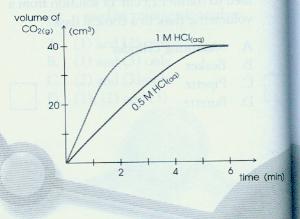
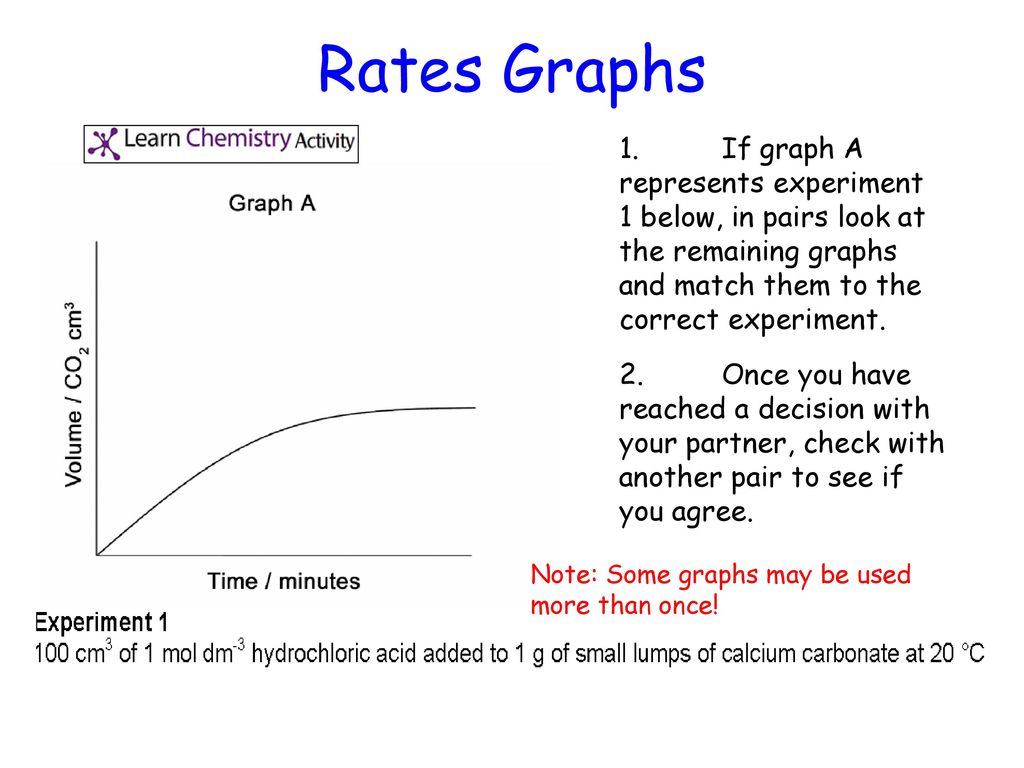
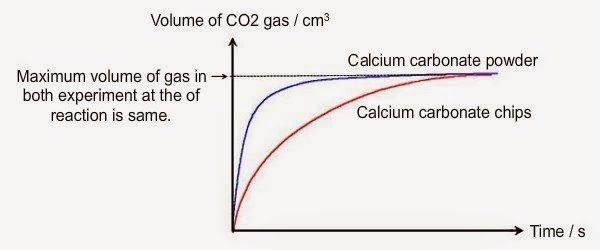
Marble chips and hydrochloric acid graph.
Hydrochloric acid to react with the marble chips independent variable marble chips to react with the acid dependent variable stopwatch to accurately time the experiment spatula to handle the marble chips measuring cylinder to precisely measure out different concentrations of hydryochloric acid electric balance to measure the mass g of the marble chips bung.
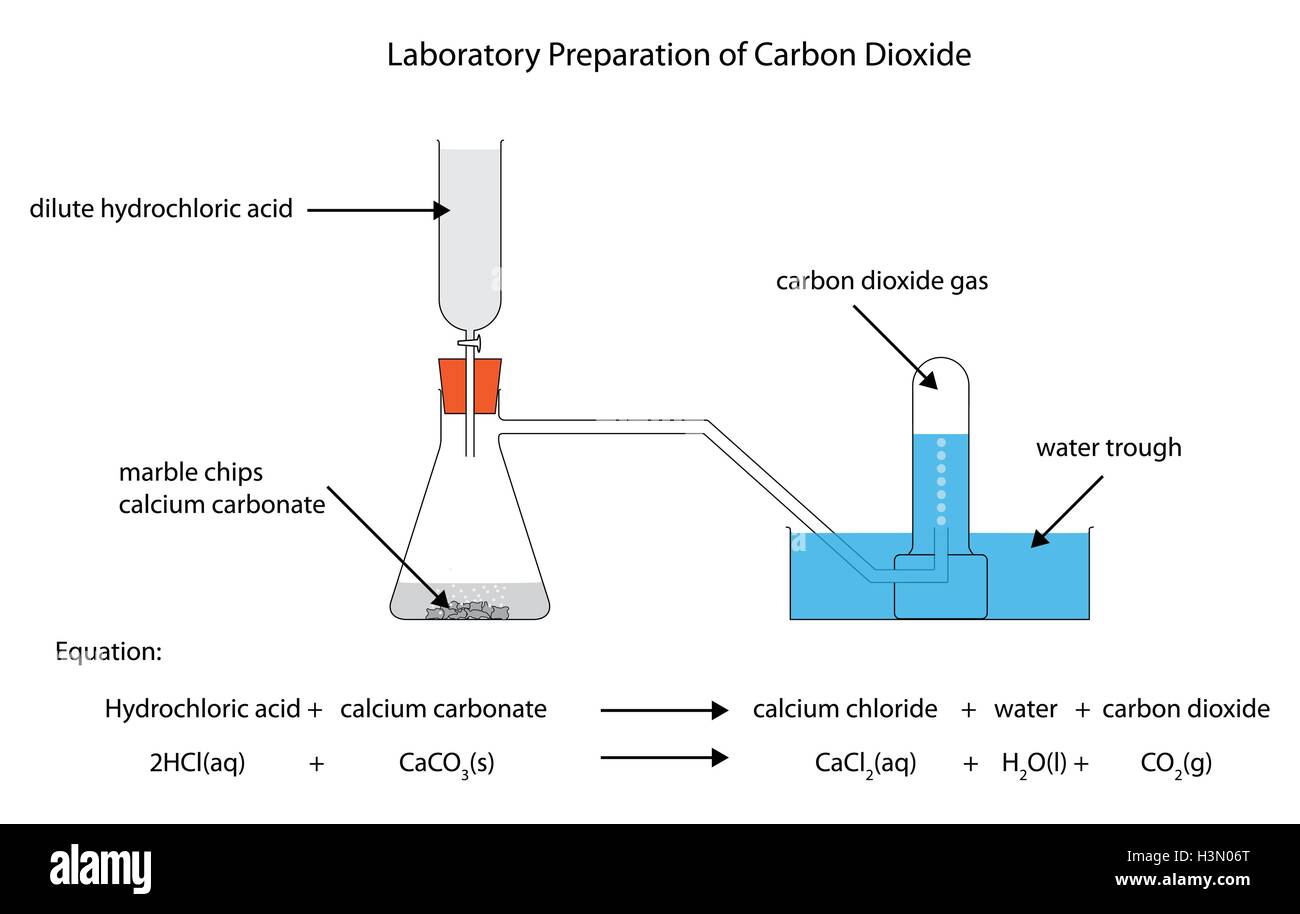
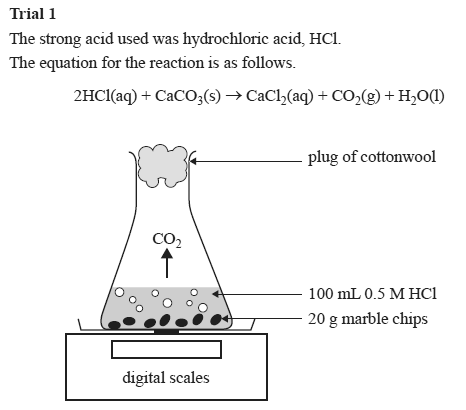
Investigating the rate of reaction between marble chips calcium carbonate and hydrochloric acid aim.
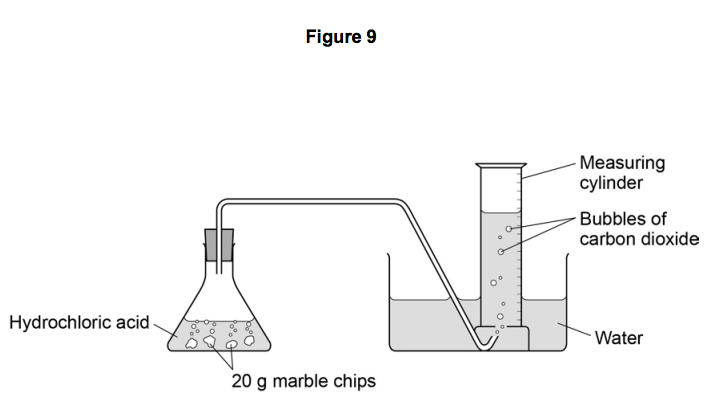
A conical flask contains the marble chips hydrochloric acid and the water that will make the reaction.
A stand to hold up the measuring cylinder.
A tube to connect the conical flask to the measuring cylinder.
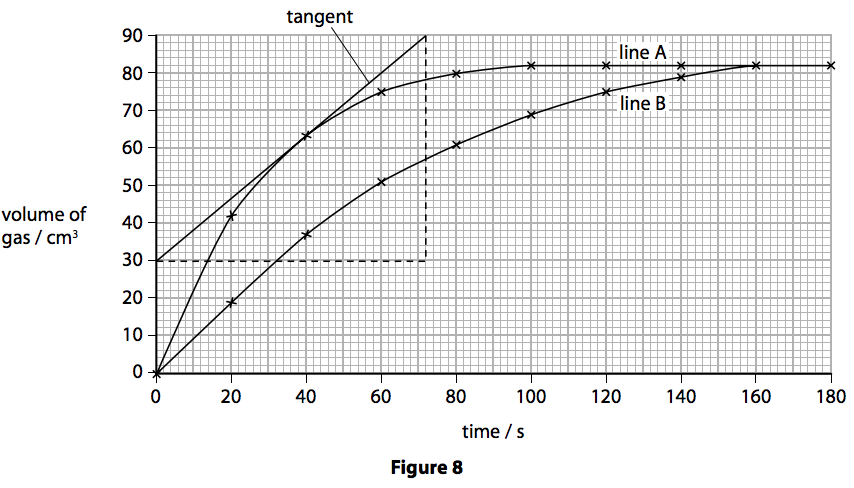
The rate of this reaction can be changed by changing the size of the marble chips.
There are many variables that affect.
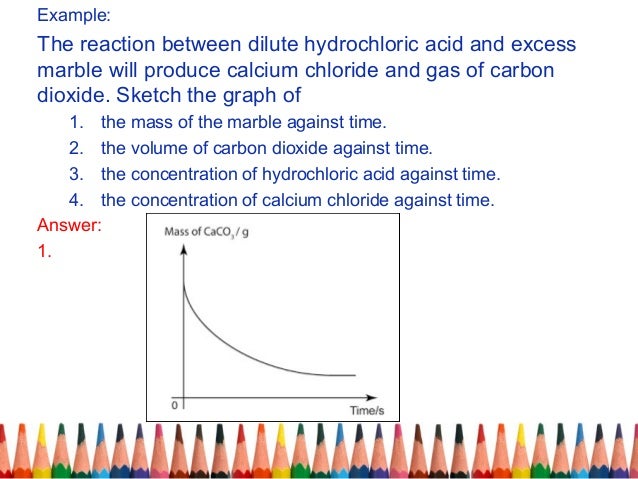
Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas.
Hydrochloric acid marble chips the experiment the aim of this experiment is to find out how different variables affect the rate at which the reaction between marble chips caco and hydrochloric acid hcl takes place.
The variables that i shall be changing will be the concentration of hydrochloric acid and water.










.jpg)